Precautionary Allergen Labeling: Informing Consumers or Just Covering Your Assets?

If you’ve been to a grocery store lately, you’ve seen the labels: “May contain [allergenic food]” or “Produced in facilities where [allergenic food] has been processed”. But what’s behind a “may contain” label? Is it an honest, science-based assessment of risk designed to inform the consumer, or is it a legalistic overreaction designed to immunize a food producer from possible lawsuits. Here, Romer Labs Senior Research Scientist Adrian Rogers introduces the VITAL® Program, an initiative of the Australia and New Zealand-based Allergen Bureau and its approach to providing realistic risk assessment that food producers can use to help their consumers make scientifically informed decisions.
Published on:
Food Allergens

All of us who buy and consume pre-packed food cannot help but notice what seems to be a proliferation of “may contain” statements on ingredients labels these days. Such precautionary allergen labelling (PAL) is voluntary on the part of the food manufactures and grocery stores and aims to guide an allergenic consumer as to the risk of potential contamination of the food from one or more food allergens during production and handling. A growing concern among the allergy community is that “may contain” statements are descending into mere alibi labelling, whereby food producers and retailers are listing all allergens to protect themselves against any possible litigation.
This is an issue of real consequence: millions of consumers around the world suffer both from the risk of allergic reactions to certain foods (including anaphylactic shock) and the limits that this risk imposes on their choice of food at the store and in a restaurant. Such precautionary labelling can severely – and unnecessarily – limit the choices of food safe to eat for an allergic consumer.
So what can be done to make the use of precautionary allergen labelling a scientific, risk-based assessment rather then something that seems only to protect manufacturers and retailers? This is the quandary that the Australian and New Zealand-based Allergen Bureau faced. The Allergen Bureau was established in 2005 as a non-profit industry organisation in partnership with national and multinational food manufacturing and marketing companies, suppliers, importers, exporters, retailers and consumer groups. The overall aim of the Allergen Bureau is to share information and experience within the food industry on the management of food allergens to ensure that consumers receive relevant, consistent and comprehensible information on food allergens.
Towards more precise risk assessment with the VITAL® Program
In consultation with multiple experts, the Allergen Bureau manages the Voluntary Incidental Trace Allergen Labelling (VITAL®) Program. While continuing to invest in VITAL® and other allergen management resources, the Allergen Bureau engages in a range of food allergen management initiatives on behalf of its stakeholders. The aim of the VITAL® Program is to make sure that manufactured food is safe to eat for the vast majority of food allergic consumers by providing consistent precautionary labelling criteria to allow allergic consumers and those who care for them to avoid buying foods that that may present a risk to the individual. In this way, they work to preserve the value of precautionary labelling as a risk management tool.
VITAL® provides a common approach to due diligence for identification, reduction and control of cross-contact allergens and the process that can determine the appropriate use of precautionary allergen labelling. Prior to any implementation of VITAL®, a robust allergen management plan must already be in place. VITAL® is designed to complement existing food safety systems such as those based on the Hazard Analysis Critical Control Point (HACCP) food safety program. The VITAL® Program determines the allergen status of incoming ingredients, including the presence of any allergens that may be accidently added through the supply chain by means of shared harvesting, storage and processing facilities. VITAL® stipulates that the allergen status be assessed product-by-product rather than by factory or line. Each ingredient and processing line is assessed for potential cross-contact allergens so that such cross-contact can be reduced to a minimum level.
A key capability of the VITAL® Program is the calculation of the concentration of allergenic protein from cross-contact allergens in the food product. The protein concentration is compared with scientifically determined levels that decide whether a “may be present” statement is recommended. This is intended as a precautionary statement advising the consumer with an allergy or intolerance to avoid the food; it is intended to be used only as a result of appropriate risk-based assessments. In cases in which cross-contact allergens cannot be eliminated, VITAL® provides a consistent process and method of communication concerning cross-contact risk. When applied correctly, the VITAL® Program, can decrease the need for PAL and, in turn, increase the choice of food available to the allergic consumer.
Creating food allergen reference doses
In recognition of the need for VITAL® to be based on sound and robust science, in 2011 the Allergen Bureau invited scientists from around the world specialising in allergen management, food allergy and risk assessment to form the VITAL® Scientific Expert Panel (VSEP). The objective of the panel was to review the underpinning science around food allergen thresholds.
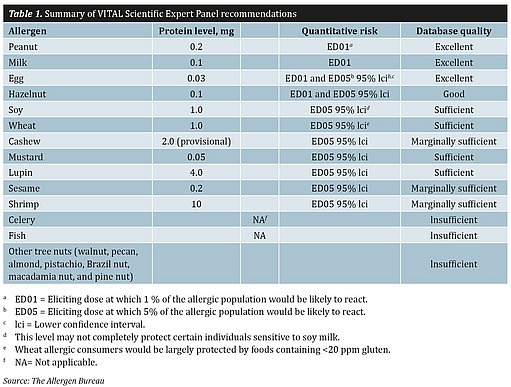
The VSEP reviewed the data from clinical (low-dose oral) food challenges from both published and unpublished studies. The papers were sourced from Australia, the United States and the European Union, and over 1800 clinical data points were collated. The data included in the review were required to meet defined quality criteria using a dose-distribution modelling approach so that the results were predictive for the entire population; this ensures that the resulting allergen thresholds were statistically sound. The derived dose-response curves enable the identification of an eliciting dose (ED) of an allergen at which a proportion of the allergic population would be likely to react (see Table 1). It is important to note, however, that this modelling does not identify a dose below which no allergic individual would react.
As a result of the work of the VSEP, the key concept of reference doses was developed. A reference dose is expressed as milligrams of total protein from the allergenic food below which only the most sensitive individual (between 1% and 5% of the allergic population, depending on the quality of the data) is likely to experience an adverse reaction. Data from large numbers of subjects were available for peanut, milk, egg, and hazelnut. Smaller amounts of individual threshold data were found for soybean, wheat, cashew, mustard, lupin, sesame seed and shrimp. There were insufficient data points available for celery, and no data points available for molluscs, therefore no reference dose for celery or molluscs have been included in the VITAL® Program to date. As there was insufficient data around (fin) fish, the Allergen Bureau adopted the original data from the US FDA Threshold Working Group. It establishes a Lowest Observed Adverse Effect Level (LOAL) of 1 mg protein and applies a 10-fold safety factor, which, when used in combination with the reference amount, allows for the determination of an action level.
Conclusion: the role of allergen analysis in the VITAL® process.
Allergen analysis plays a significant role in the application of VITAL®. However, it is only one part of the overall process of risk assessment. It is intended to provide additional information to help inform the assessment rather than to be used as a stand-alone tool.
Analysis can assist in several crucial areas: verifying ingredient allergen statements, verifying the allergen profile of raw materials and potential raw material cross-contact, targeting the analysis to assess cleaning efficacy and cleaning validations, confirming assumptions made during the risk assessment process, monitoring the effect of critical changes, and validating the VITAL® risk assessment.
Analysis is complex and thus needs to be matrix- and allergen-specific. It is important to consider the nature of the food or surface under analysis and the processes to which it has been exposed to ensure that the method applied is appropriate. When comparing analytical test results with concentrations calculated from a VITAL® risk assessment, it is important to ensure that the units of measurement are comparable.
The VITAL® action levels grid uses the concentration (ppm) of total protein. However, analytical results may be expressed in a range of units and calibrators. Methods used should be robust, reliable, reproducible, sensitive, and specific; an appropriate sampling plan is crucial.
The quantitative assessment of cross-contact allergens needs to take the sporadic nature of cross-contact allergens into account by applying a comprehensive testing regime. Allergen analysis can be used to validate the assumptions used for the allergen management plan as well as results found using physical assessment.
It is important that the science behind VITAL® remains current, transparent and relevant to all stakeholders. To enable this, the Allergen Bureau continues to engage with the VSEP, the industry and research groups and is constantly reviewing allergen threshold data. To this end, an updated iteration of the VITAL® Program, VITAL® 3.0 will be launching very soon.
Through the continued adoption of the VITAL® Program and the use of other science-based allergen risk assessment processes by the food industry, one can hope that the misunderstanding and confusion currently surrounding precautionary allergen labelling can begin to be resolved. Rather than benefiting the manufacturers and retailers, PAL should become a trusted method that empowers allergic consumers to make safer food choices to stop preventable fatalities while not artificially limiting their choices as consumers.