What is Zearalenone?
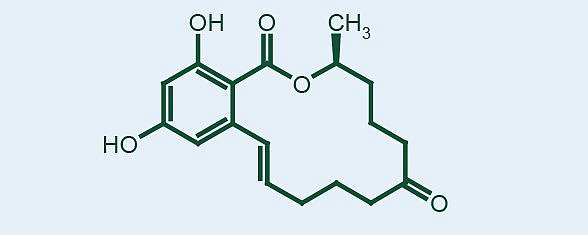
This compound is chemically a phenolic resorcyclic acid lactone that is primarily an estrogenic fungal metabolite. It is observed on thin layer chromatographic plates under short wavelength ultraviolet light as a greenish fluorescent compound. This compound may be produced in concert with deoxynivalenol by certain isolates of the fungus.
Production and occurrence
The major species of fungus responsible for producing this mycotoxin is Fusarium graminearum. In some of the older literature this organism is called F. roseum. Grain infected with this organism often will have a pink color because of a pigment that may be simultaneously produced with the zearalenone.
Most often the compound is found in corn, however, it is found also in other important crops such as wheat, barley, sorghum and rye throughout various countries of the world. In wheat the conditions for the occurrence of zearalenone would be essentially the same as for the occurrence of deoxynivalenol as the organism gains entry into the host plant in the same manner. Generally, the Fusarium species grow in moist cool conditions and similarly invade crops under these more favorable conditions. As noted above, the same organism produces both of these compounds. This same organism is capable of producing both compounds in corn. The finding of aflatoxin co-occurring with zearalenone and deoxynivalenol would imply that infection was established by two different fungi, Aspergillus flavus in the case of aflatoxin and F. graminearum in the case of the latter two mycotoxins. In wheat, sorghum and corn, it is well-established that zearalenone occurs in preharvest grain but in other commodities the surveys are insufficient to determine if the zearalenone occurred pre- or postharvest.
Variations in the incidence of zearalenone occur with different crop years, cereal crop and perhaps geographical areas.
As with other fungi, to avoid growth of F. graminearum in grains during storage the moisture level should be <14%. Perhaps, zearalenone can be produced in relatively cool conditions compared to some other mycotoxins but it is likely that most grains mentioned above can become contaminated with zearalenone during storage and levels that were present in the grain preharvest may increase if the grain is not sufficiently dried and stored.
Toxicity
The most notable effect of zearalenone is that it causes precocious development of mammae and other estrogenic effects in young gilts as well as prepucial enlargement in young barrows.
Swine appear to be the animals most significantly affected and are considerably more sensitive than rodents. Weak piglets and small litter size have been attributed to the effects of zearalenone when fed to sows during gestation. Levels of 0.5 to 1.0 ppm of dietary zearalenone have been associated with the latter effects while hyperestrogenism in swine was associated with dietary levels of 1.5 to 5.0 ppm. Twelve ppm zearalenone was found in sorghum that was involved in bovine abortion. Zearalenone appears to bind to estrogen receptors and can result in hormonal changes. Zearalenone does not appear to be involved in mortalities because of its high oral LD50. Interestingly, zearalenone or its metabolites have been suspected to cause precocious pubertal changes in young children in Puerto Rico. The occurrence of this phenomenon in other countries needs confirmation as to the causation. Of note is that the metabolite of zearalenone known as α-zearalenol, is actually more estrogenic than is the parent compound (Richardson et al., 1985).
Published on:
Mycotoxin