Microbiology
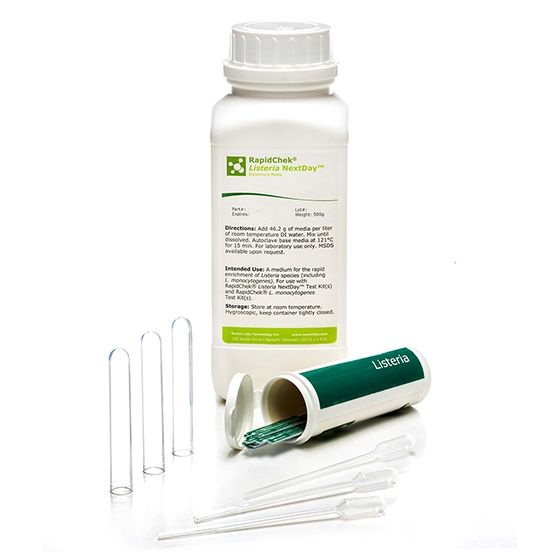
RapidChek®Listeria Nextday™ Environmental Sampling System
SH-Listeria Nextday Environmental Sampling SystemMicrobiology Pathogenic bacteria LFD Qualitative
The RapidChek®Listeria NextDay™ test system is one of the first testing systems for all Listeria strains which is AOAC approved. The proprietary enrichment media needs no additional supplements to offer next day test results. Time to result: 24 - 48 hours (depending on the analyzed sample). Environmental sampling system includes Pur-Blue DUO™ sampling devices with pre-made RapidChek ®Listeria NextDay™ media.- Analyte: Listeria species
- Technology/Application: LFD
- Result type: Qualitative

RapidChek®Listeria Media
SH-Listeria MediaMicrobiology Pathogenic bacteria LFD Qualitative
The RapidChek®Listeria media offers simple preparation of enrichment media without autoclaving and enables fast and flexible test planning. Supplement is stored at 2C - 8C. Note: pre-made liquid media is made to order. Lead times may vary.- Analyte: Listeria species
- Technology/Application: LFD
- Result type: Qualitative
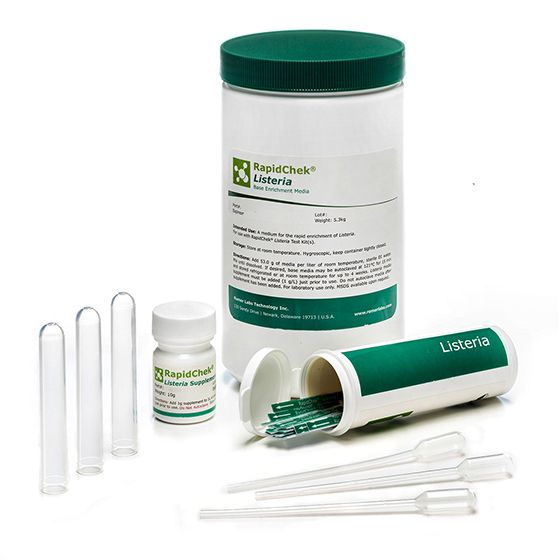
RapidChek®Listeria Food System
SH-Listeria Food SystemMicrobiology Pathogenic bacteria LFD Qualitative
The RapidChek®Listeria test system offers a single-step enrichment media to enable fast, accurate and simple Listeria detection within 40 hours. The simple preparation of enrichment media without autoclaving offers fast and flexible test planning. Time to result: 40 - 48 hours (depending on the analyzed sample). The supplement is stored at 2C - 8C.- Analyte: Listeria species
- Technology/Application: LFD
- Result type: Qualitative
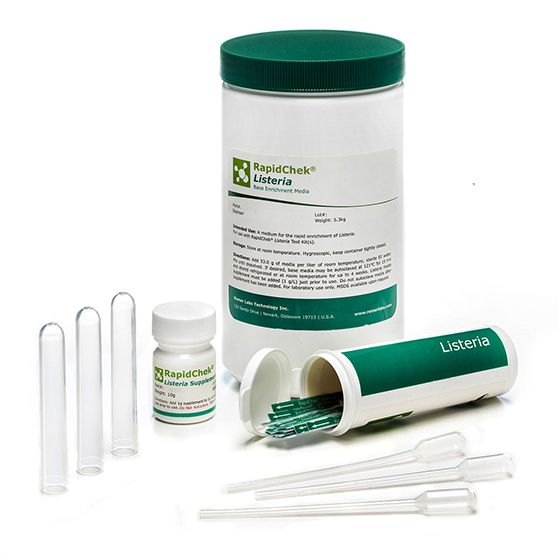
RapidChek®Listeria Environmental System
SH-Listeria Environmental SystemMicrobiology Pathogenic bacteria LFD Qualitative
The RapidChek®Listeria test system offers a single-step enrichment media to enable fast, accurate and simple Listeria detection within 40 hours. The simple preparation of enrichment media without autoclaving offers fast and flexible test planning. Time to result: 40 - 48 hours (depending on the analyzed sample). The supplement is stored at 2C-8C.- Analyte: Listeria species
- Technology/Application: LFD
- Result type: Qualitative

RapidChek®Listeria monocytogenes strips
SH-Listeria monocytogenes stripsMicrobiology Pathogenic bacteria LFD Qualitative
The RapidChek® Listeria monocytogenes test system is one of the first one-step immunological AOAC-certified testing system for Listeria monocytogenes. The proprietary RapidChek® Listeria NextDay™ enrichment media, also used in the Listeria NextDay™ systems, offers a short enrichment time.- Analyte: Listeria monocytogenes
- Technology/Application: LFD
- Result type: Qualitative
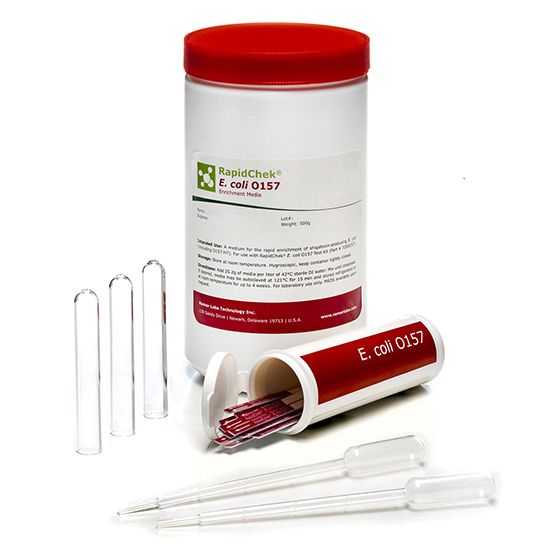
RapidChek®E. coli O157 Test System
SH-E. coli Test SystemMicrobiology Pathogenic bacteria LFD Qualitative
RapidChek®E. coli O157 test system offers an easy to prepare one-step enrichment media, without the need of additional supplements, combined with proprietary antibodies to detect the pathogenic E. coli serotype O157 (incl. H7) in various foodstuff within a minimum of 8 hours. Time to result: 8 - 18 hours (depending on the analyzed sample)- Analyte: E. coli O157 (including H7)
- Technology/Application: LFD
- Result type: Qualitative

RapidChek®E. coli O157 Strips
SH-E. coli StripsMicrobiology Pathogenic bacteria LFD Qualitative
RapidChek®E. coli O157 test strip with proprietary antibodies to detect the pathogenic E. coli serotype O157 (incl. H7) in various foodstuff within a minimum of 8 hours.- Analyte: E. coli O157 (including H7)
- Technology/Application: LFD
- Result type: Qualitative

RapidChek®Campylobacter Strips
SH-Campylobacter-StripsMicrobiology Pathogenic bacteria LFD Qualitative
The RapidChek®Campylobacter test strips can be used with RapidChek®Campylobacter media (for 47 - 49 h time to result).- Analyte: C. jejuni, C. coli, C. lari
- Technology/Application: LFD
- Result type: Qualitative

RapidChek®Campylobacter Media
SH-Campylobacter-MediaMicrobiology Pathogenic bacteria LFD Qualitative
The RapidChek®Campylobacter proprietary enrichment media needs no additional supplements. Media is added directly to sterile water and ready for use. Samples are incubated aerobically for a simplified enrichment procedure.- Analyte: C. jejuni, C. coli, C. lari
- Technology/Application: LFD
- Result type: Qualitative

Plastic Boot Cover
SH-PBCMicrobiology Indicator microorganisms Pathogenic bacteria Spoilage microorganisms Primary Production
Plastic Boot Covers to wear over boots or shoes to avoid cross contamination between sampling sites- Technology/Application: Primary Production
- Product/Environment: Environmental Monitoring
- Storage Temperature: 15°C - 25°C