SH-Salmonella Enteriditis System
RapidChek® SELECT™Salmonella Enteritidis System
Microbiology Pathogenic bacteria LFD Qualitative
Sign-in or register for more informationHave a question or need support?
Do you have questions about our products and/or services? Contact us below.
Contact usDescription & Properties
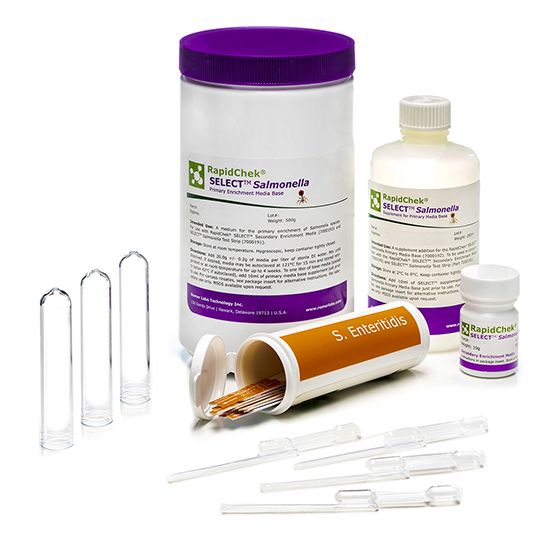
The RapidChek® SELECT™ SE test is one of the first FDA awarded Test Method Equivalent and AOAC approved commercially available, rapid, sero-specific assay. It uses the same proprietary media system as the RapidChek® SELECT™ Salmonella test kit. The difference is made by the highly specific Salmonella serogroup D1 antibody on the lateral flow device. Supplement is stored at 2C - 8C.
| Microbiology | Pathogenic bacteria |
|---|---|
| Technology/Application | LFD |
| Storage Temperature | 15°C - 25°C |
| Result type | Qualitative |
| Product/Environment | Environmental Monitoring, Product Testing |
| Approvals | AOAC, FDA |
Materials
Materials supplied in the standard product
- strips
- pipettes
- tubes
- media
Materials required but not supplied
- sample bags
- balance
- incubator
Documents
10001398 (7000222):
10001399 (7000223):
10001714 (7000220P):
10001715 (7000220S):





