Shop
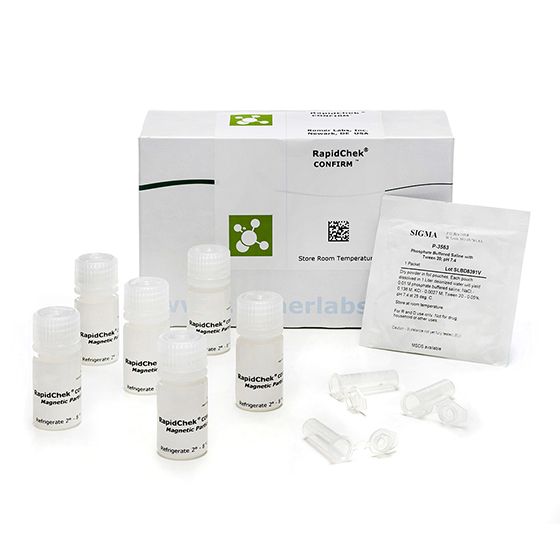
RapidChek® SELECT™Salmonella Enteritidis Confirmation System
SH-Salmonella Enteriditis Confirmation SystemMicrobiology Pathogenic bacteria LFD Qualitative
The use of the RapidChek® CONFIRM™ SE IMS kit reduces the presence of other non-SE, Salmonella species through washing steps and concentrates the Group D1 species.- Analyte: Salmonella Enteriditis
- Technology/Application: LFD
- Result type: Qualitative
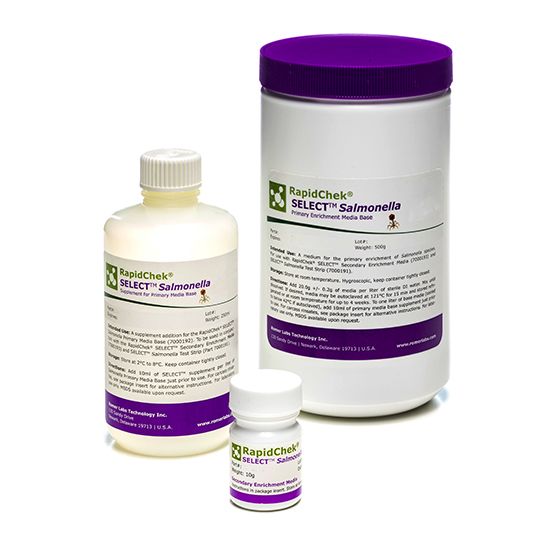
RapidChek® SELECT™Salmonella Enteriditis Media
SH-Salmonella Enteriditis MediaMicrobiology Pathogenic bacteria LFD Qualitative
The RapidChek® SELECT™ Salmonella Enteritidis media combines the patented primary phage media with a highly productive secondary enrichment to enable fast, accurate and simple Salmonella Enteritidis detection. Supplement is stored at 2C - 8C. Note: pre-made liquid media is made to order. Lead times may vary.- Analyte: Salmonella species including Salmonella Enteriditis
- Technology/Application: LFD
- Result type: Qualitative

RapidChek® E. coli O157 Media
SH-E. coli MediaMicrobiology Pathogenic bacteria LFD Qualitative
RapidChek®E. coli O157 is a one-step rapid enrichment media for shigatoxin-producing E. coli (including O157:H7).- Analyte: Shigatoxin producing E. coli (Including O157:H7)
- Technology/Application: LFD
- Result type: Qualitative
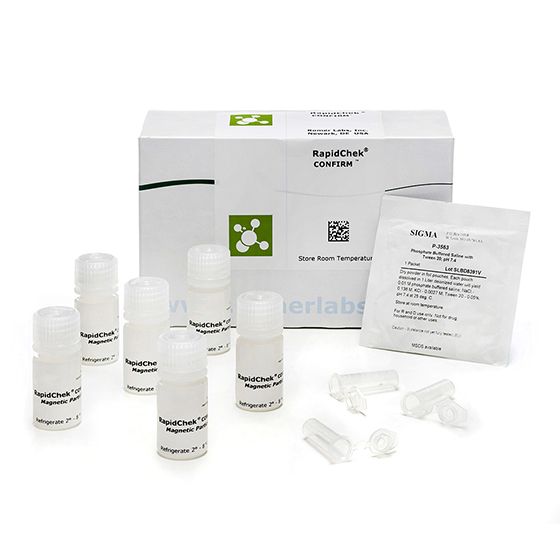
RapidChek® CONFIRM™ STEC Confirmation Reagents
SH-STEC Confirmation ReagentsMicrobiology Pathogenic bacteria LFD Qualitative
The RapidChek® CONFIRM™ non-O157 STEC IMS confirmation kit uses specific, high-affinity antibodies, each attached to separate magnetic particles. Should any of the non-O157 STECs be present, it will bind to the magnetic particles via the antibody. When the sample is plated to selective agar for confirmation, E. coli O26, O45, O103, O111, O121, and O145 are the predominant colonies that form, increasing the likelihood of picking that organism from a plate.- Technology/Application: LFD
- Result type: Qualitative
- Product/Environment: Environmental Monitoring, Product Testing
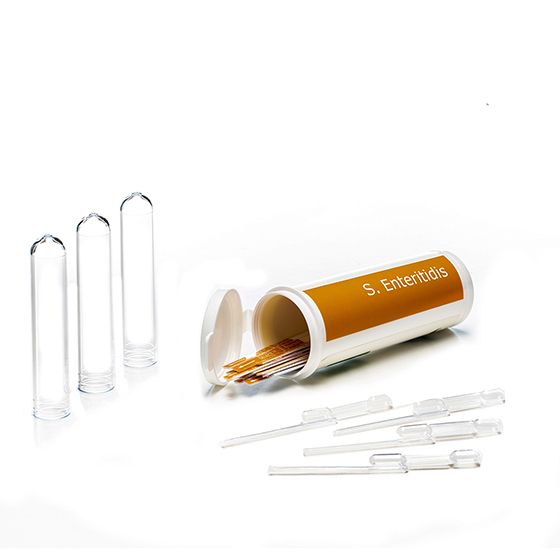
RapidChek®Salmonella Enteriditis Strips
SH-Salmonella Enteriditis StripsMicrobiology Pathogenic bacteria LFD Qualitative
The RapidChek® SELECT™ SE test is one of the first FDA awarded Test Method Equivalent and AOAC approved commercially available, rapid, sero-specific assay. There is a highly specific Salmonella serogroup D1 antibody on the lateral flow device.- Analyte: Salmonella Enteritidis
- Technology/Application: LFD
- Result type: Qualitative
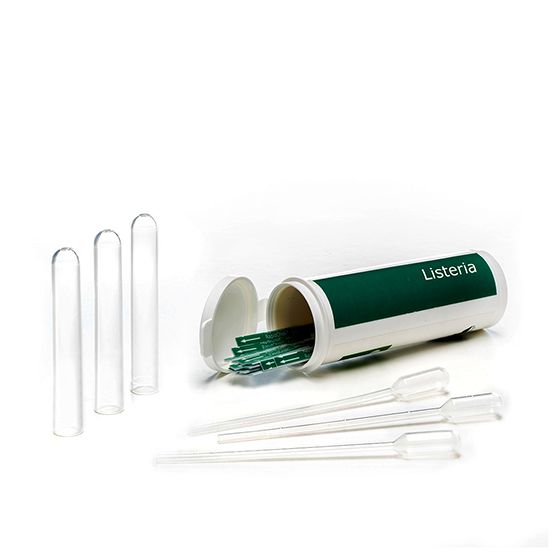
RapidChek®Listeria Strips
SH-Listeria StripsMicrobiology Pathogenic bacteria LFD Qualitative
The RapidChek®Listeria test strips can be used with RapidChek®Listeria NextDay media (for 24 - 40h time to result) or RapidChek®Listeria media system (for 40 - 48h time to result). Strips are available as single tests or comb format (8 tests per comb).- Analyte: Listeria species
- Technology/Application: LFD
- Result type: Qualitative

RapidChek®Listeria Nextday™ Media
SH-Listeria Nextday MediaMicrobiology Pathogenic bacteria LFD Qualitative
The RapidChek®Listeria NextDay™ proprietary enrichment media needs no additional supplements to offer next day test results. Note: pre-made liquid media is made to order. Lead times may vary.- Analyte: Listeria species
- Technology/Application: LFD
- Result type: Qualitative
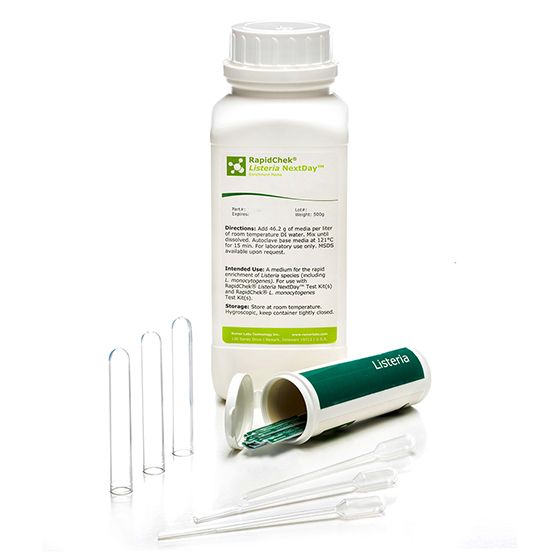
RapidChek®Listeria NextDay™ Food System
SH-Listeria Nextday Food SystemMicrobiology Pathogenic bacteria LFD Qualitative
The RapidChek®Listeria NextDay™ test system is one of the first testing systems for all Listeria strains which is AOAC approved. The proprietary enrichment media needs no additional supplements to offer next day test results. Time to result: 27 - 48 hours (depending on the analyzed sample)- Analyte: Listeria species
- Technology/Application: LFD
- Result type: Qualitative
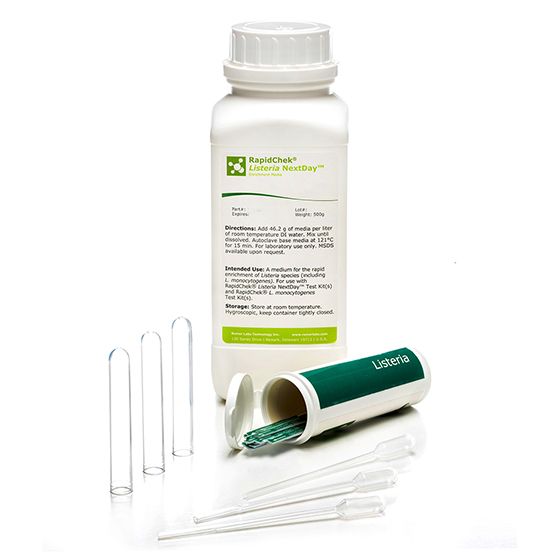
RapidChek®Listeria Nextday™ Environmental System
SH-Listeria Nextday Environmental SystemMicrobiology Pathogenic bacteria LFD Qualitative
The RapidChek®Listeria NextDay™ test system is one of the first testing systems for all Listeria strains which is AOAC approved. The proprietary enrichment media needs no additional supplements to offer next day test results. Time to result: 24 - 48 hours (depending on the analyzed sample).- Analyte: Listeria species
- Technology/Application: LFD
- Result type: Qualitative
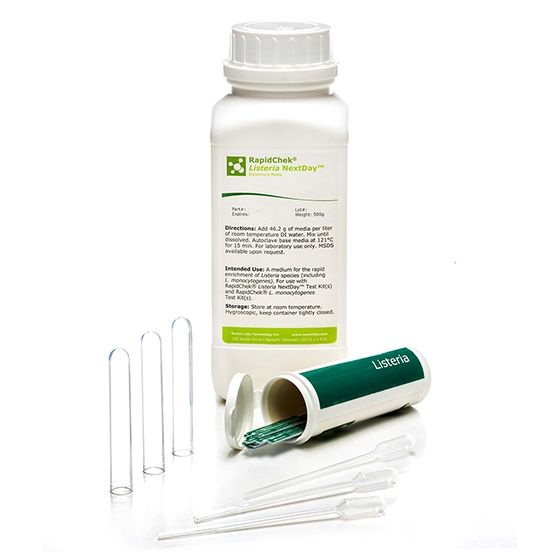
RapidChek®Listeria Nextday™ Environmental Sampling System
SH-Listeria Nextday Environmental Sampling SystemMicrobiology Pathogenic bacteria LFD Qualitative
The RapidChek®Listeria NextDay™ test system is one of the first testing systems for all Listeria strains which is AOAC approved. The proprietary enrichment media needs no additional supplements to offer next day test results. Time to result: 24 - 48 hours (depending on the analyzed sample). Environmental sampling system includes Pur-Blue DUO™ sampling devices with pre-made RapidChek ®Listeria NextDay™ media.- Analyte: Listeria species
- Technology/Application: LFD
- Result type: Qualitative